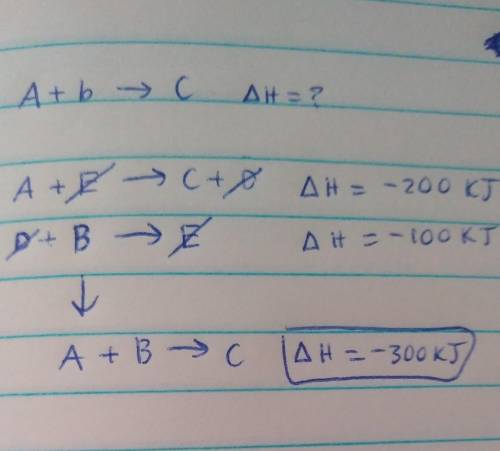Chemistry, 05.07.2021 23:20 anavallesdemiguel2
Determine the enthalpy for the reaction A+B --> C If we know the following:
A+E --> C+D; delta H = -200 kJ
D+B --> E; delta H = -100 kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1


Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
Determine the enthalpy for the reaction A+B --> C If we know the following:
A+E --> C+D; delt...
Questions


Biology, 27.07.2019 23:30






Social Studies, 27.07.2019 23:30





Mathematics, 27.07.2019 23:30

History, 27.07.2019 23:30

Mathematics, 27.07.2019 23:30