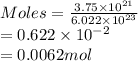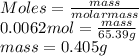Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3


Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
Determine the mass in grams of 3.75 x 10^21 atoms of zinc. (the mass of one mole of zinc is 65.39 g)...
Questions





Mathematics, 22.02.2021 19:50



Mathematics, 22.02.2021 19:50



Mathematics, 22.02.2021 19:50


Mathematics, 22.02.2021 19:50

English, 22.02.2021 19:50




Mathematics, 22.02.2021 19:50


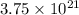 atoms of zinc is 0.405 g.
atoms of zinc is 0.405 g.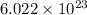 atoms. So, the number of moles in given number of atoms is as follows.
atoms. So, the number of moles in given number of atoms is as follows.