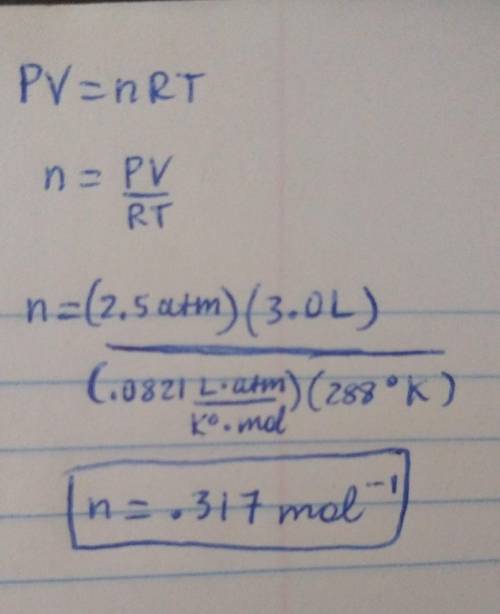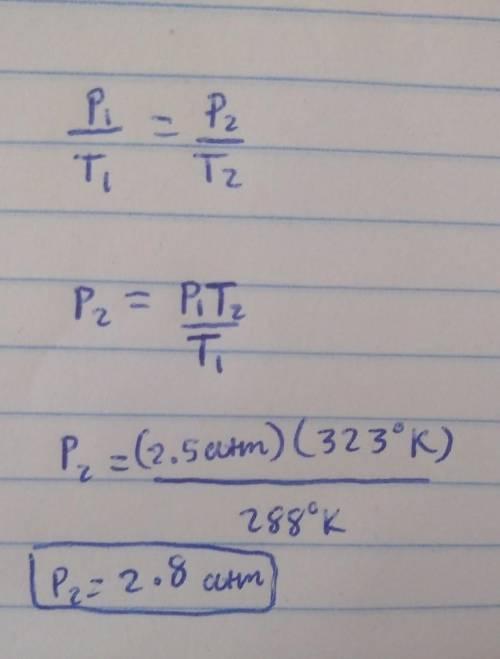Chemistry, 03.07.2021 01:30 emilylunaa
A 3.0-liter sample of an ideal gas is at a pressure of 2.5 atm at 15oC. (i) How many moles of gas are in the sample? (ii) If the volume does not change, what is the pressure of the gas when the temperature is 50oC?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
A 3.0-liter sample of an ideal gas is at a pressure of 2.5 atm at 15oC. (i) How many moles of gas ar...
Questions

Biology, 18.11.2020 05:10

Mathematics, 18.11.2020 05:10

Mathematics, 18.11.2020 05:10

Mathematics, 18.11.2020 05:10

Mathematics, 18.11.2020 05:10

History, 18.11.2020 05:10


English, 18.11.2020 05:10

Mathematics, 18.11.2020 05:10

History, 18.11.2020 05:10

English, 18.11.2020 05:10

Mathematics, 18.11.2020 05:10

French, 18.11.2020 05:10



Mathematics, 18.11.2020 05:10




History, 18.11.2020 05:20