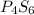Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1
You know the right answer?
Compound X has a molar mass of 316.25 g*mol^-1 and the following composition:
element & mass %<...
Questions

History, 09.12.2019 03:31



Mathematics, 09.12.2019 03:31



Mathematics, 09.12.2019 03:31



Chemistry, 09.12.2019 03:31

Biology, 09.12.2019 03:31

Mathematics, 09.12.2019 03:31

Mathematics, 09.12.2019 03:31



Mathematics, 09.12.2019 03:31



Physics, 09.12.2019 03:31

History, 09.12.2019 03:31