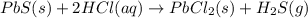Chemistry, 01.07.2021 21:10 cristinaledford3696
Solid lead(II) sulfide reacts with aqueous hydrochloric acid to form solid lead(II) chloride and dihydrogen sulfide gas. Express your answer as a chemical equation.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2


Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
Solid lead(II) sulfide reacts with aqueous hydrochloric acid to form solid lead(II) chloride and dih...
Questions

Mathematics, 19.03.2021 18:40

Mathematics, 19.03.2021 18:40


Mathematics, 19.03.2021 18:40

Physics, 19.03.2021 18:40


Social Studies, 19.03.2021 18:40

Mathematics, 19.03.2021 18:40

Biology, 19.03.2021 18:40

Mathematics, 19.03.2021 18:40

Mathematics, 19.03.2021 18:40


Mathematics, 19.03.2021 18:40

Mathematics, 19.03.2021 18:40

Mathematics, 19.03.2021 18:40


Mathematics, 19.03.2021 18:40

Social Studies, 19.03.2021 18:40


Social Studies, 19.03.2021 18:40