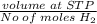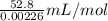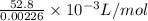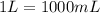Chemistry, 01.07.2021 16:30 izearaholland7308
Calculate the Experimental Molar Volume in L/mol of the Hydrogen gas, H2, if the volume of H2 at STP is 52.8 mL and the mass of Magnesium metal, Mg, used in the experiment is 0.055 g.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2


Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
Calculate the Experimental Molar Volume in L/mol of the Hydrogen gas, H2, if the volume of H2 at STP...
Questions

Mathematics, 18.03.2021 05:10

Mathematics, 18.03.2021 05:10

Mathematics, 18.03.2021 05:10

Mathematics, 18.03.2021 05:10

Chemistry, 18.03.2021 05:10






Business, 18.03.2021 05:10


Computers and Technology, 18.03.2021 05:10



Chemistry, 18.03.2021 05:10

Mathematics, 18.03.2021 05:10

Mathematics, 18.03.2021 05:10


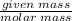
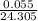 moles
moles