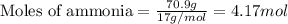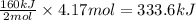2NH3(g)→N2(g)+3H2(g)

Chemistry, 01.07.2021 15:40 rowdycar313p0ao5k
A chemist measures the energy change
ΔH during the following reaction:
2NH3(g)→N2(g)+3H2(g)
ΔH=160kJUse the information to answer the following questions. This reaction is:.
a. endothermic
b. exothermic
Suppose 70.9 g of NH3 react. Will any heat be released or absorbed?
a. Yes, absorbed
b. Yes, released
c. No.
If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2


Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
A chemist measures the energy change
ΔH during the following reaction:
2NH3(g)→N2(g)+3H2(g)
2NH3(g)→N2(g)+3H2(g)
Questions

Mathematics, 09.12.2019 03:31









English, 09.12.2019 03:31


Mathematics, 09.12.2019 03:31


Mathematics, 09.12.2019 03:31

English, 09.12.2019 03:31

Mathematics, 09.12.2019 03:31




Mathematics, 09.12.2019 03:31

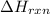 is positive for these reactions.
is positive for these reactions.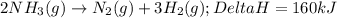
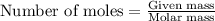 ......(1)
......(1)