
Chemistry, 29.06.2021 19:00 reagancunningham2004
The equilibrium constant (K p) for the interconversion of PCl 5 and PCl 3 is 0.0121:
PCl5 (g) → PCl3 (g) + Cl2 (g)
A vessel is charged with PCl 5 giving an initial pressure of 0.123 atm and yields PCl 3 and Cl 2. At equilibrium, the partial pressure of PCl 3 is atm.
A) 0.0782.
B) 0.0455.
C) 0.0908.
D) 0.0330.
E) 0.123.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
The equilibrium constant (K p) for the interconversion of PCl 5 and PCl 3 is 0.0121:
PCl5 (g) → PCl...
Questions

Mathematics, 19.11.2020 23:30



Geography, 19.11.2020 23:30


Social Studies, 19.11.2020 23:30

Health, 19.11.2020 23:30

Computers and Technology, 19.11.2020 23:30

Mathematics, 19.11.2020 23:30

Mathematics, 19.11.2020 23:30

Spanish, 19.11.2020 23:30



Mathematics, 19.11.2020 23:30

English, 19.11.2020 23:30



Mathematics, 19.11.2020 23:30

Chemistry, 19.11.2020 23:30

English, 19.11.2020 23:30

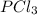 is 0.0330 atm.
is 0.0330 atm.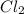 . Hence, let us assume that x quantity of
. Hence, let us assume that x quantity of 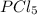 is decomposed and gives x quantity of
is decomposed and gives x quantity of 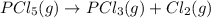
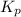 of this reaction is as follows.
of this reaction is as follows.![K_{p} = \frac{[PCl_{3}][Cl_{2}]}{[PCl_{5}]}\\0.0121 = \frac{x \times x}{(0.123 - x)}\\x = 0.0330](/tpl/images/1386/0346/b037d.png)


