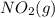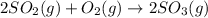The second reaction in the formation of sulfuric acid occurs slowly.
2 upper S upper O subscript 2 (g) plus upper O subscript 2 (g) right arrow 2 upper S upper O subscript 3 (g).
NO2 is added to the reaction to speed it up. In which form would this substance be a homogeneous catalyst for this reaction?
NO2(g)
NO2(l)
NO2(s)
NO2(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
The second reaction in the formation of sulfuric acid occurs slowly.
2 upper S upper O subscript 2...
Questions




Mathematics, 12.10.2021 07:50



World Languages, 12.10.2021 07:50


Mathematics, 12.10.2021 07:50




Mathematics, 12.10.2021 07:50




Mathematics, 12.10.2021 07:50


Biology, 12.10.2021 07:50