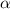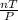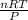Chemistry, 28.06.2021 18:20 Naysa150724
The expression below was formed by combining different gas laws. V is proportional to StartFraction n T over P EndFraction. Which law was used to determine the relationship between the volume and the number of moles in this equation?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
You know the right answer?
The expression below was formed by combining different gas laws. V is proportional to StartFraction...
Questions


Mathematics, 29.09.2020 14:01


English, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01

Chemistry, 29.09.2020 14:01





Geography, 29.09.2020 14:01

Physics, 29.09.2020 14:01

English, 29.09.2020 14:01


History, 29.09.2020 14:01


English, 29.09.2020 14:01