
Chemistry, 25.06.2021 14:00 maggie9459
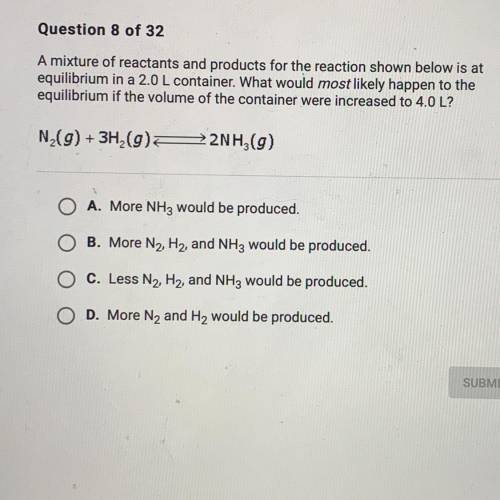
A mixture of reactants and products for the reacting shown below is at equilibrium 2.0 L container. What would most likely happen to the equilibrium if the volume of the container were increased to 4.0 L?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1


Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
A mixture of reactants and products for the reacting shown below is at equilibrium 2.0 L container....
Questions

Arts, 05.10.2019 22:30

English, 05.10.2019 22:30




Mathematics, 05.10.2019 22:30


History, 05.10.2019 22:30

Mathematics, 05.10.2019 22:30

History, 05.10.2019 22:30

Advanced Placement (AP), 05.10.2019 22:30

History, 05.10.2019 22:30

Mathematics, 05.10.2019 22:30

Physics, 05.10.2019 22:30




Social Studies, 05.10.2019 22:30


Mathematics, 05.10.2019 22:30



