
Chemistry, 25.06.2021 05:20 gudon986732
A chemist must prepare 900.0 mL of potassium hydroxide solution with a pH of 12.20 at 25°C.
He will do this in three steps:
• Fill a 900.0 mL volumetric flask about halfway with distilled water.
• Weigh out a small amount of solid potassium hydroxide and add it to the flask.
. Fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits and put your answer in grams (g).

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
A chemist must prepare 900.0 mL of potassium hydroxide solution with a pH of 12.20 at 25°C.
He will...
Questions



Mathematics, 06.07.2021 16:50


Mathematics, 06.07.2021 16:50

Mathematics, 06.07.2021 17:00



Mathematics, 06.07.2021 17:00


World Languages, 06.07.2021 17:00



Chemistry, 06.07.2021 17:00


Mathematics, 06.07.2021 17:00

Social Studies, 06.07.2021 17:00

Chemistry, 06.07.2021 17:00

Mathematics, 06.07.2021 17:00

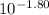 = 0.0158 M
= 0.0158 M


