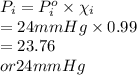171 g of sucrose ( MW of 342, melting point 186 oC, boiling point very high, and vapor pressure is negligible) is dissolved in one liter of water at 25 oC. At 25 oC the vapor pressure of water is 24 mmHg. Which value is closest to the vapor pressure (VP) of this solution at 25 oC?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1



Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
171 g of sucrose ( MW of 342, melting point 186 oC, boiling point very high, and vapor pressure is n...
Questions





Computers and Technology, 12.01.2021 01:40

Mathematics, 12.01.2021 01:40

History, 12.01.2021 01:40


Mathematics, 12.01.2021 01:40


History, 12.01.2021 01:40

Mathematics, 12.01.2021 01:40


Business, 12.01.2021 01:40


Mathematics, 12.01.2021 01:40


Mathematics, 12.01.2021 01:40


Business, 12.01.2021 01:40

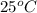 is closest to the value 24 mm Hg.
is closest to the value 24 mm Hg.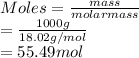
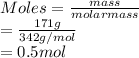
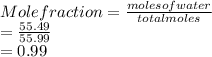
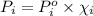
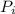 = vapor pressure of component i over the solution
= vapor pressure of component i over the solution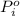 = vapor pressure of pure component i
= vapor pressure of pure component i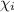 = mole fraction of i
= mole fraction of i