Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1


Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
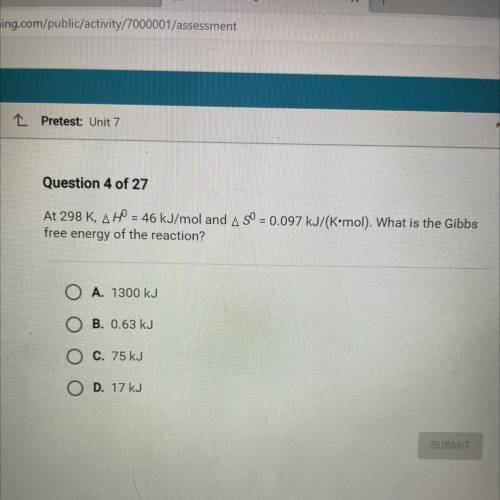
At 298 K, AH = 46 kJ/mol and A SO = 0.097 kJ/(K•mol). What is the Gibbs
free energy of the reaction...
Questions

Mathematics, 27.03.2020 02:45

Computers and Technology, 27.03.2020 02:45


Mathematics, 27.03.2020 02:45

Mathematics, 27.03.2020 02:45



Mathematics, 27.03.2020 02:45

History, 27.03.2020 02:45


English, 27.03.2020 02:45


Mathematics, 27.03.2020 02:46




English, 27.03.2020 02:46



Mathematics, 27.03.2020 02:47