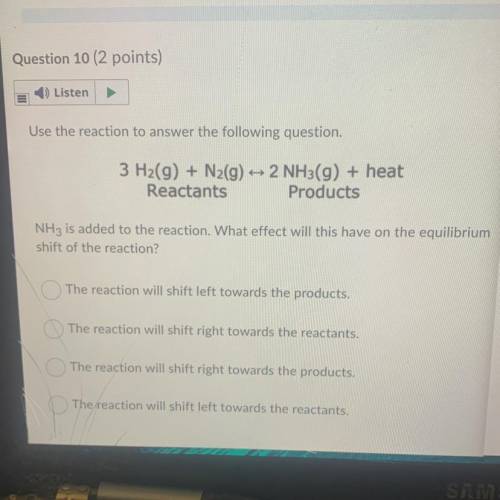
Use the reaction to answer the following question.
3 H2(g) + N2(g) + 2 NH3(g) + heat
Reactant...

Use the reaction to answer the following question.
3 H2(g) + N2(g) + 2 NH3(g) + heat
Reactants Products
NH3 is added to the reaction. What effect will this have on the equilibrium
shift of the reaction?
The reaction will shift left towards the products.
The reaction will shift right towards the reactants.
The reaction will shift right towards the products.
The reaction will shift left towards the reactants.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
Questions


Mathematics, 17.05.2021 01:00


Social Studies, 17.05.2021 01:00

Mathematics, 17.05.2021 01:00



Mathematics, 17.05.2021 01:00

Mathematics, 17.05.2021 01:00


Biology, 17.05.2021 01:00

Social Studies, 17.05.2021 01:00

Mathematics, 17.05.2021 01:00



Mathematics, 17.05.2021 01:00



Mathematics, 17.05.2021 01:00

English, 17.05.2021 01:00



