
Chemistry, 24.06.2021 22:00 aryannaholmes9
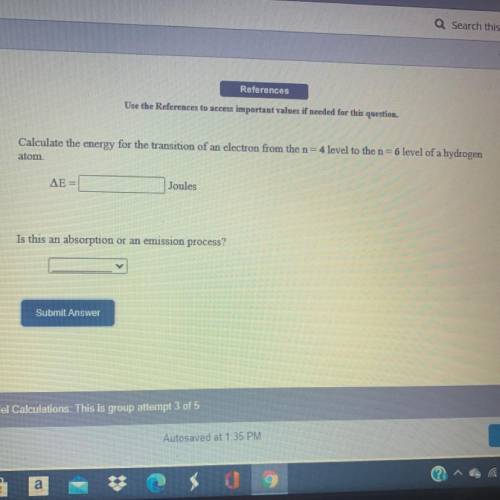
Calculate the energy for the transition of an electron from the n=4 level to the n=6 level of a hydrogen atom.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
Calculate the energy for the transition of an electron from the n=4 level to the n=6 level of a hydr...
Questions


Mathematics, 01.11.2020 01:00


Mathematics, 01.11.2020 01:00

Mathematics, 01.11.2020 01:00



English, 01.11.2020 01:00

English, 01.11.2020 01:00


History, 01.11.2020 01:00

Mathematics, 01.11.2020 01:00

Mathematics, 01.11.2020 01:00

Business, 01.11.2020 01:00

Mathematics, 01.11.2020 01:00







