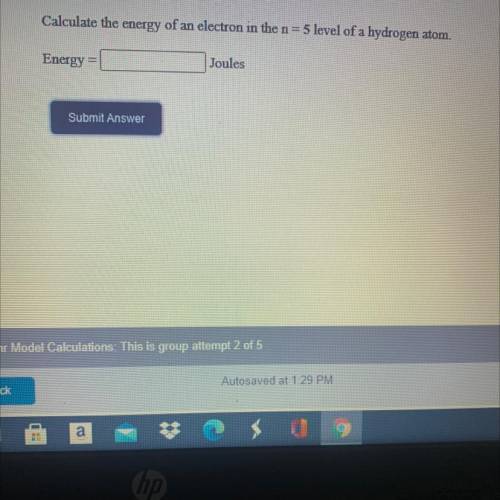
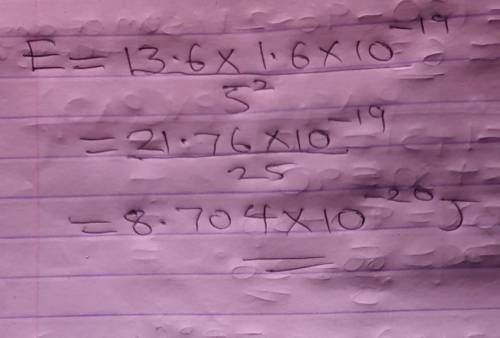Calculate the energy of an electron in the n = 5 level of a hydrogen atom.
...


Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1
You know the right answer?
Questions


History, 26.08.2021 01:50

Computers and Technology, 26.08.2021 01:50




English, 26.08.2021 01:50

Mathematics, 26.08.2021 01:50


Social Studies, 26.08.2021 02:00

Biology, 26.08.2021 02:00

Mathematics, 26.08.2021 02:00



Spanish, 26.08.2021 02:00



Mathematics, 26.08.2021 02:00

Spanish, 26.08.2021 02:00