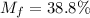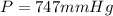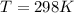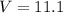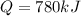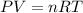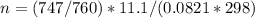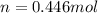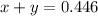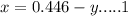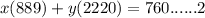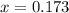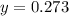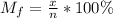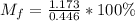Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
A gaseous fuel mixture stored at 747 mmHg and 298 K contains only methane (CH4) and propane (C3H8)....
Questions








Mathematics, 12.06.2020 00:57


Mathematics, 12.06.2020 00:57

Mathematics, 12.06.2020 00:57

Advanced Placement (AP), 12.06.2020 00:57






Engineering, 12.06.2020 00:57

Mathematics, 12.06.2020 00:57

English, 12.06.2020 00:57