
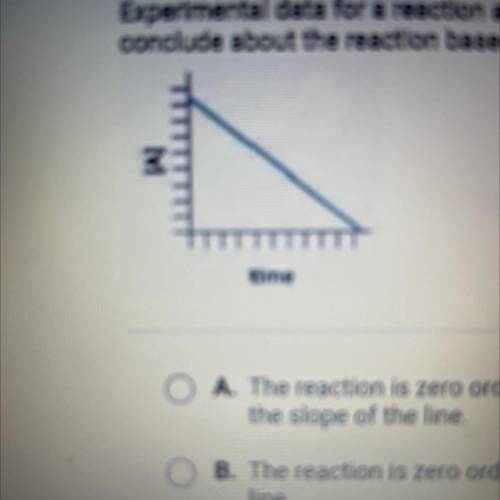
Experimental data for a reaction are collected and graphed. What can you
conclude about the reaction based on the graph?
A. The reaction is zero order, and the rate constant is the negative of
the slope of the line
B. The reaction is zero order, and the rate constant is the slope of the
line
C. The reaction is first order, and the rate constant is the slope of the
line,
D. The reaction is first order, and the rate constant is the negative eg

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2


Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
Experimental data for a reaction are collected and graphed. What can you
conclude about the reactio...
Questions


English, 17.09.2021 19:20


English, 17.09.2021 19:20




History, 17.09.2021 19:20



History, 17.09.2021 19:20


Mathematics, 17.09.2021 19:20






History, 17.09.2021 19:20



