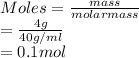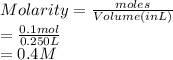Chemistry, 24.06.2021 08:20 justapointie
define a mole of a substancea standard solution of naoh is prepared by dissolving 4 g of the dilute in 250cm3 determine the molarity of the solution

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1


Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
define a mole of a substancea standard solution of naoh is prepared by dissolving 4 g of the dilute...
Questions

Mathematics, 24.11.2020 20:40

Advanced Placement (AP), 24.11.2020 20:40


French, 24.11.2020 20:40

Mathematics, 24.11.2020 20:40




Mathematics, 24.11.2020 20:40


Biology, 24.11.2020 20:40

Mathematics, 24.11.2020 20:40

Mathematics, 24.11.2020 20:40


History, 24.11.2020 20:40


Mathematics, 24.11.2020 20:40


Mathematics, 24.11.2020 20:40

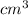 (1
(1