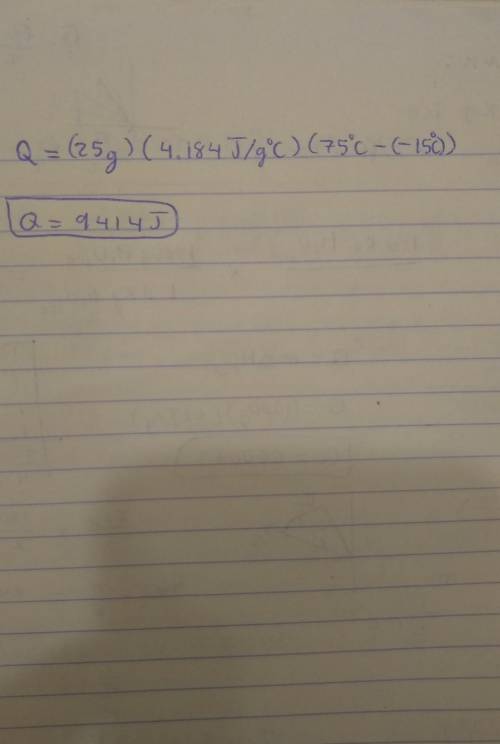Chemistry, 24.06.2021 03:30 sevaramirabell
Note: Please show all work and calculation setups to get full credit. T. he following may be used on this assignment: specific heat of (water=4.184 J/g oC; ice=2.03 J/g oC; steam=1.99 184 J/g oC); heat of fusion of water=80. cal/g; heat of vaporization=540 cal/g; 1cal=4.184J. Calculate the energy required (in J) to convert 25 g of ice at -15 oC to water at 75 oC.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
Note: Please show all work and calculation setups to get full credit. T. he following may be used on...
Questions






Mathematics, 20.10.2020 22:01








Mathematics, 20.10.2020 22:01

Biology, 20.10.2020 22:01

English, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01


Computers and Technology, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01