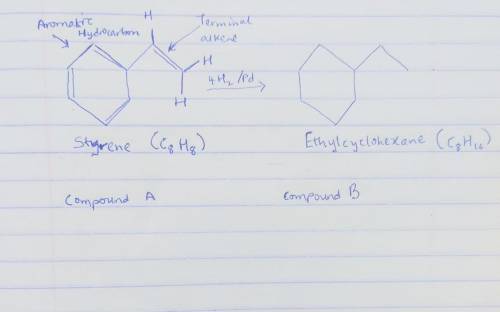
Compound A has the formula C8H8. It reacts rapidly with acidic KMnO4 but reacts with only 1 equivalent of H2 over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, A reacts with 4 equivalents of H2, and hydrocarbon B, C8H16, is produced. The reaction of A with KMnO4 gives CO2 and a carboxylic acid C, C7H6O2.
Required:
Draw the structure of compound B below.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

You know the right answer?
Compound A has the formula C8H8. It reacts rapidly with acidic KMnO4 but reacts with only 1 equivale...
Questions

History, 21.12.2021 17:20


Mathematics, 21.12.2021 17:20





Social Studies, 21.12.2021 17:20

English, 21.12.2021 17:20

SAT, 21.12.2021 17:20





Social Studies, 21.12.2021 17:20

Mathematics, 21.12.2021 17:20


English, 21.12.2021 17:20


English, 21.12.2021 17:30