Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1


Chemistry, 23.06.2019 12:10
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1
You know the right answer?
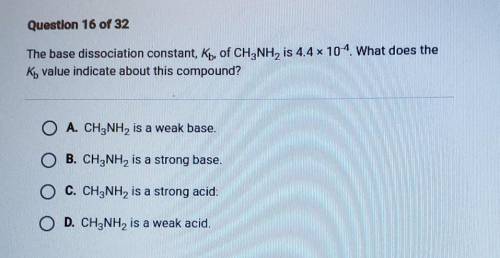
The base dissociation constant, Kb, of CH3NH2 is 4.4x 10-4. What does the Ky value indicate about th...
Questions


Mathematics, 20.11.2020 08:00


Mathematics, 20.11.2020 08:00

Biology, 20.11.2020 08:00

Arts, 20.11.2020 08:00

Mathematics, 20.11.2020 08:00








Physics, 20.11.2020 08:00

Mathematics, 20.11.2020 08:00




World Languages, 20.11.2020 08:00