

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

You know the right answer?
Consider the following intermediate chemical equations.
CH2(g) →C(s) + 2H2(g)
CC1.(g) → C(s)...
CC1.(g) → C(s)...
Questions

Arts, 12.05.2021 20:30

Mathematics, 12.05.2021 20:30

Chemistry, 12.05.2021 20:30

Mathematics, 12.05.2021 20:30

Arts, 12.05.2021 20:30

Computers and Technology, 12.05.2021 20:30

History, 12.05.2021 20:30






Mathematics, 12.05.2021 20:30

Mathematics, 12.05.2021 20:30

English, 12.05.2021 20:30


Mathematics, 12.05.2021 20:30

Mathematics, 12.05.2021 20:30

Mathematics, 12.05.2021 20:30

Mathematics, 12.05.2021 20:30

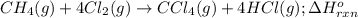

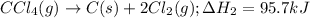

![\Delta H^o_{rxn}=[1\times (\Delta H_1)] + [1\times (-\Delta H_2)] + [2\times (\Delta H_3)]](/tpl/images/1382/1138/89421.png)
![\Delta H^o_{rxn}=[1\times (74.6)] + [1\times (-95.7)] + [2\times (-92.3)]\\\\\Delta H^o_{rxn}=-205.7kJ](/tpl/images/1382/1138/33de2.png)


