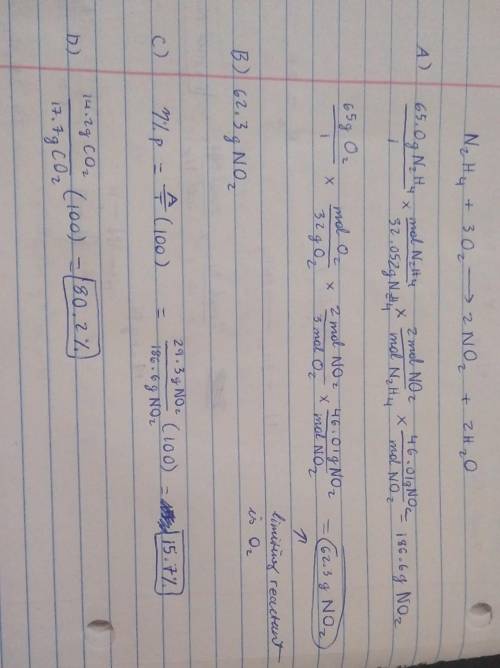19) Hydrazine, N2H4, a substance used as rocket fuel, reacts with oxygen as follows:
N2H4 (1) + 3 O2(g) → 2 NO2(g) + 2 H20 (8)
A) If 65.0 g of hydrazine are reacted with 65.0 g of oxygen, which is the limiting reactant?
usg of Oz X Imol
.
B) How many grams of NO2 are produced from the limiting reactant in part A?
C) If 29.3 g of NO2 are obtained from the reaction in Part A, what is the percent yield?
20) What is the percent yield if 14.2 g of CO2 were produced and 17.7 g were calculated to be
produced from the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1


Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
19) Hydrazine, N2H4, a substance used as rocket fuel, reacts with oxygen as follows:
N2H4 (1) + 3 O...
Questions