Buffer Calculation Questions
um Question 14 ( points)
Calculate the pH of a buffer that conta...

Chemistry, 22.06.2021 17:10 lilyrockstarmag
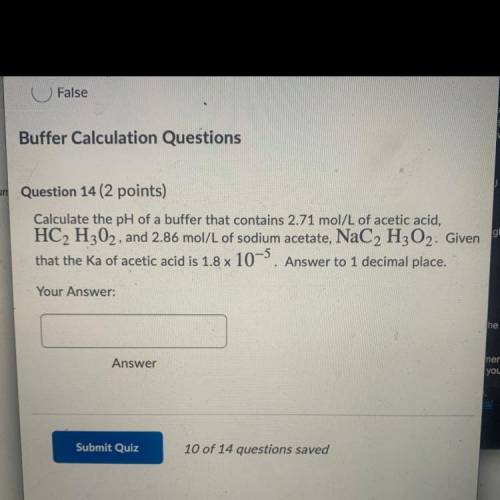
Buffer Calculation Questions
um Question 14 ( points)
Calculate the pH of a buffer that contains 2.71 mol/L of acetic acid,
HC2 H302, and 2.86 mol/L of sodium acetate, NaC2 H2O2. Given
that the Ka of acetic acid is 1.8 x 10-5. Answer to 1 decimal place.
Your
Answer

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2



Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
Questions


Mathematics, 20.01.2021 23:10

English, 20.01.2021 23:10




English, 20.01.2021 23:10


Mathematics, 20.01.2021 23:10


Biology, 20.01.2021 23:10

Mathematics, 20.01.2021 23:10

Mathematics, 20.01.2021 23:10






Mathematics, 20.01.2021 23:10

Business, 20.01.2021 23:10



