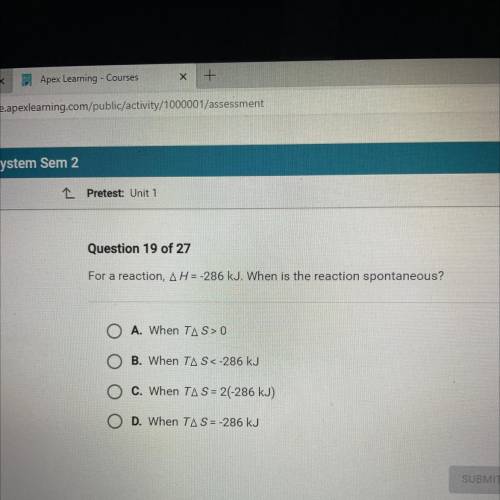
For a reaction, AH = -286 kJ. When is the reaction spontaneous?
O A. When TAS> 0
O B. When...

Chemistry, 22.06.2021 14:50 lebron06james
For a reaction, AH = -286 kJ. When is the reaction spontaneous?
O A. When TAS> 0
O B. When TAS<-286 kJ
O C. When TA S = 2(-286 kJ)
O D. When TAS = -286 kJ

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
Questions


Health, 12.10.2020 01:01


Mathematics, 12.10.2020 01:01

Physics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01



Physics, 12.10.2020 01:01







Computers and Technology, 12.10.2020 01:01


English, 12.10.2020 01:01

Social Studies, 12.10.2020 01:01

History, 12.10.2020 01:01



