
Chemistry, 22.06.2021 04:40 bearminar2156
2 NH3 + 3 CuO --> 3 Cu + N2 + 3 H2O
In the above equation, how many moles of N2 can be made when 113.6 grams of CuO are consumed?
Round your answer to the nearest tenth. If your answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect. :
Element Molar Mass
Hydrogen
1
Nitrogen
14
Copper
63.5
Oxygen
16

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

You know the right answer?
2 NH3 + 3 CuO --> 3 Cu + N2 + 3 H2O
In the above equation, how many moles of N2 can be made when...
Questions


Biology, 13.12.2020 08:40

History, 13.12.2020 08:40

English, 13.12.2020 08:40

Mathematics, 13.12.2020 08:40


Chemistry, 13.12.2020 08:40

Mathematics, 13.12.2020 08:40

History, 13.12.2020 08:40




Arts, 13.12.2020 08:40


History, 13.12.2020 08:40

Mathematics, 13.12.2020 08:40

Biology, 13.12.2020 08:40

Chemistry, 13.12.2020 08:40


English, 13.12.2020 08:40

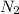 are produced in the reaction
are produced in the reaction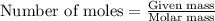 ......(1)
......(1)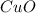 = 113.6 g
= 113.6 g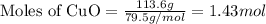
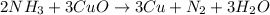
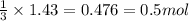 of
of 

