Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
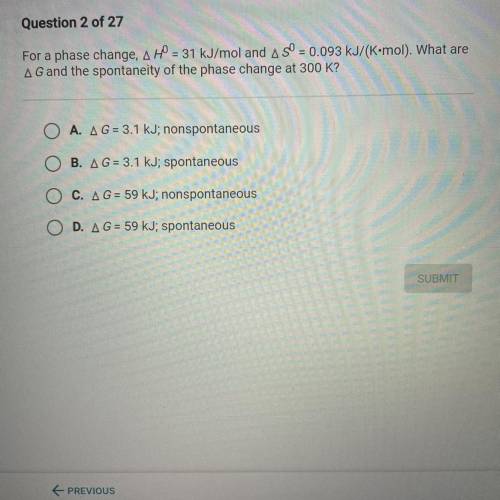
For a phase change, A H0 = 31 kJ/mol and A S0 = 0.093 kJ/(K•mol). What are
A G and the spontaneity...
Questions



English, 28.11.2021 20:40


Health, 28.11.2021 20:40

Mathematics, 28.11.2021 20:40

Social Studies, 28.11.2021 20:40

Chemistry, 28.11.2021 20:40

Mathematics, 28.11.2021 20:40

Mathematics, 28.11.2021 20:40

Mathematics, 28.11.2021 20:40


Mathematics, 28.11.2021 20:40

Mathematics, 28.11.2021 20:40

Computers and Technology, 28.11.2021 20:40

Mathematics, 28.11.2021 20:40

Mathematics, 28.11.2021 20:40

Chemistry, 28.11.2021 20:40

Mathematics, 28.11.2021 20:40

Mathematics, 28.11.2021 20:40