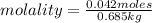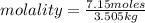Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1


Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
Calculate the molality of each of the following solutions:
(a) 14.3 g of sucrose (C12H22O11) in 685...
Questions

Computers and Technology, 19.03.2021 20:40

Mathematics, 19.03.2021 20:40




Mathematics, 19.03.2021 20:40


Mathematics, 19.03.2021 20:40


Chemistry, 19.03.2021 20:40





History, 19.03.2021 20:40




History, 19.03.2021 20:40

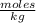
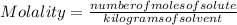
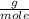 , then 14.3 grams of the compound represents the following number of moles:
, then 14.3 grams of the compound represents the following number of moles: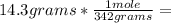 0.042 moles
0.042 moles