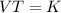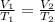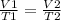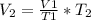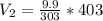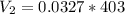Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1


Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
If 9.9L of helium are in a tire at 303k, how many liters will be present at 403k if the pressure is...
Questions



Biology, 22.04.2020 01:08


Computers and Technology, 22.04.2020 01:09

Geography, 22.04.2020 01:09



Computers and Technology, 22.04.2020 01:09

Physics, 22.04.2020 01:09





Mathematics, 22.04.2020 01:09


Mathematics, 22.04.2020 01:09

Mathematics, 22.04.2020 01:09


Mathematics, 22.04.2020 01:09