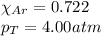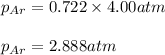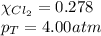Chemistry, 21.06.2021 16:00 benjamenburton1
At 50.0 oC, a reinforced tank contains 675.5 grams of gaseous argon and 465.0 g of gaseous molecular chlorine with a total pressure of 4.00 atm. Calculate the following:
a. How many moles of Ar are in the tank?
b. How many moles of Cl, are in the tank?
c. Total moles of gas in the tank.
d. The mole fraction of Ar.
e. The mole fraction of Cl2.
f. The Partial Pressure of Ar.
g. The Partial Pressure of Cl2.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1

Chemistry, 23.06.2019 13:30
If a fast moving car making a loud noise approaches and moves past the person what will happen as the distance between the two increases?
Answers: 1

Chemistry, 24.06.2019 01:00
Watch the video to learn about the process of hydrogen bonding. hydrogen bonding is a type of intermolecular force that exists between polar covalent molecules, one of which has a hydrogen atom that is bonded to a small and extremely electronegative element, specifically an n, o, or f atom, on the other molecule. hydrogen bonding is a subset of dipole-dipole forces.
Answers: 1
You know the right answer?
At 50.0 oC, a reinforced tank contains 675.5 grams of gaseous argon and 465.0 g of gaseous molecular...
Questions


Geography, 03.08.2019 07:00


Biology, 03.08.2019 07:00

Mathematics, 03.08.2019 07:00



English, 03.08.2019 07:00

History, 03.08.2019 07:00

Mathematics, 03.08.2019 07:00

Mathematics, 03.08.2019 07:00


Mathematics, 03.08.2019 07:00

Mathematics, 03.08.2019 07:00






Mathematics, 03.08.2019 07:00

 is 16.94 moles
is 16.94 moles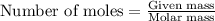 ......(1)
......(1)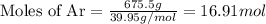
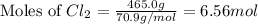
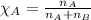 .....(2)
.....(2)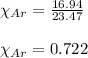
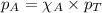 .....(3)
.....(3)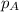 is the partial pressure of component A in the mixture and
is the partial pressure of component A in the mixture and 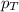 is the total partial pressure of the mixture
is the total partial pressure of the mixture