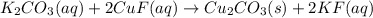Aqueous potassium carbonate was mixed with aqueous copper (1) fluoride and a crystallized copper (1) carbonate product was formed. A crystalized product is a solid. The other product, potassium fluoride, remains dissolved in solution. Consider the other product and it’s phase, and then write the balanced molecular equation for this precipitation reaction. Express your answer as a chemical equation including phases. Type an underscore (_) or a carat (^) to add subscripts and superscripted more quickly.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

You know the right answer?
Aqueous potassium carbonate was mixed with aqueous copper (1) fluoride and a crystallized copper (1)...
Questions


Advanced Placement (AP), 10.03.2021 05:30

Mathematics, 10.03.2021 05:30

Mathematics, 10.03.2021 05:30


Arts, 10.03.2021 05:30

Mathematics, 10.03.2021 05:30


Mathematics, 10.03.2021 05:30

Mathematics, 10.03.2021 05:30

Mathematics, 10.03.2021 05:30

Mathematics, 10.03.2021 05:30






Mathematics, 10.03.2021 05:30


Mathematics, 10.03.2021 05:30