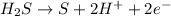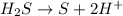Which is the balanced version of the half-reaction below?
H2S → S+H+
...

Chemistry, 21.06.2021 08:00 Tyrant4life
Which is the balanced version of the half-reaction below?
H2S → S+H+
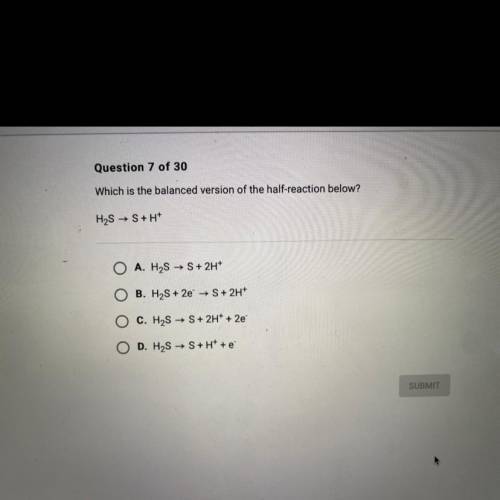

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

You know the right answer?
Questions



Geography, 18.05.2021 20:00

Mathematics, 18.05.2021 20:00


Mathematics, 18.05.2021 20:00


English, 18.05.2021 20:00


Chemistry, 18.05.2021 20:00

English, 18.05.2021 20:00



Mathematics, 18.05.2021 20:00

English, 18.05.2021 20:00

SAT, 18.05.2021 20:00




Mathematics, 18.05.2021 20:00