
Chemistry, 20.06.2021 08:30 erinleyanne
Which statement is true to explain how the system below would respond to being heated.
a)Captionless Image
b)the equilibrium will shift right toward the products.
c)the forward rate would increase more than the reverse rate
d)the reverse rate would increase more than the forward rate
e)[SO₃] will increase
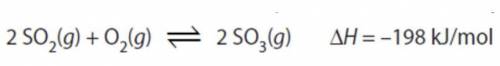

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
How many miles of calcium oxide will be produced when 1.6 miles of iron (iii) oxide react with calcium phosphate
Answers: 1

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

You know the right answer?
Which statement is true to explain how the system below would respond to being heated.
a)Captionles...
Questions




Mathematics, 26.07.2021 06:20




Mathematics, 26.07.2021 06:20



Mathematics, 26.07.2021 06:20



Mathematics, 26.07.2021 06:20


Social Studies, 26.07.2021 06:20




Mathematics, 26.07.2021 06:20



