Liquid hexane
(CH,(CH), CH) will react with gaseous oxygen (0) to produce gaseous carbon dioxide (CO2) and gaseous water (1,0). Suppose 1.72 g
of hexane is mixed with 8.0 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the
correct number of significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
Liquid hexane
(CH,(CH), CH) will react with gaseous oxygen (0) to produce gaseous carbon dioxide (C...
Questions

Computers and Technology, 01.01.2021 15:40

Social Studies, 01.01.2021 15:40

Chemistry, 01.01.2021 15:40

Business, 01.01.2021 15:40

Mathematics, 01.01.2021 15:40

Social Studies, 01.01.2021 15:40



English, 01.01.2021 15:50

Mathematics, 01.01.2021 15:50

Computers and Technology, 01.01.2021 15:50





Computers and Technology, 01.01.2021 15:50




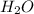 produced is 2.52 g
produced is 2.52 g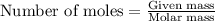 ......(1)
......(1)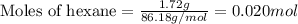
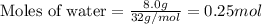
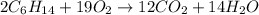
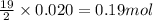 of oxygen gas
of oxygen gas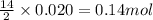 of
of