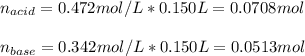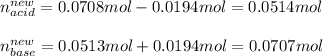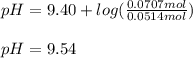Chemistry, 18.06.2021 04:10 fluffy374747
A buffer solution contains 0.472 M hydrocyanic acid and 0.342 M sodium cyanide. If 0.0194 moles of sodium hydroxide are added to 150 mL of this buffer, what is the pH of the resulting solution

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
A buffer solution contains 0.472 M hydrocyanic acid and 0.342 M sodium cyanide. If 0.0194 moles of s...
Questions

Social Studies, 05.05.2020 03:04


Mathematics, 05.05.2020 03:04



Mathematics, 05.05.2020 03:04

Mathematics, 05.05.2020 03:04


Mathematics, 05.05.2020 03:05

Biology, 05.05.2020 03:05

History, 05.05.2020 03:05




History, 05.05.2020 03:05

Mathematics, 05.05.2020 03:05