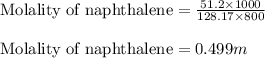Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

You know the right answer?
Calculate the molality of a solution that contains 51.2 g of naphthalene, C10H8, in 500 mL of carbon...
Questions

Mathematics, 05.07.2020 17:01


Mathematics, 05.07.2020 17:01

Mathematics, 05.07.2020 17:01










Mathematics, 05.07.2020 17:01

English, 05.07.2020 17:01




Business, 05.07.2020 17:01

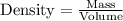 ......(1)
......(1)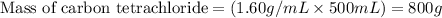
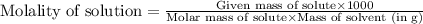 .....(2)
.....(2)