Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

You know the right answer?
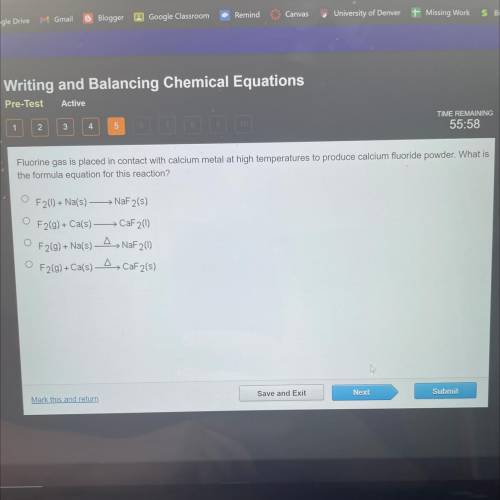
Fluorine gas is placed in contact with calcium metal at high temperatures to produce calcium fluorid...
Questions

Chemistry, 02.08.2019 20:30

Mathematics, 02.08.2019 20:30

Spanish, 02.08.2019 20:30




Spanish, 02.08.2019 20:30


History, 02.08.2019 20:30




History, 02.08.2019 20:30



Mathematics, 02.08.2019 20:30

English, 02.08.2019 20:30