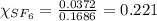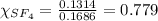Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2
You know the right answer?
A tank at is filled with of sulfur tetrafluoride gas and of sulfur hexafluoride gas. You can assume...
Questions

Mathematics, 20.01.2021 06:10



Mathematics, 20.01.2021 06:10

Mathematics, 20.01.2021 06:10

Chemistry, 20.01.2021 06:10




Mathematics, 20.01.2021 06:10

Mathematics, 20.01.2021 06:10


Mathematics, 20.01.2021 06:10

Mathematics, 20.01.2021 06:10






Mathematics, 20.01.2021 06:20

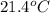 is filled with 5.43 g of sulfur hexafluoride gas and 14.2 g of sulfur tetrafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas. Round each of your answers to significant digits.
is filled with 5.43 g of sulfur hexafluoride gas and 14.2 g of sulfur tetrafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas. Round each of your answers to significant digits. ......(1)
......(1)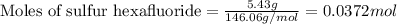
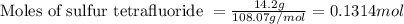
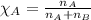 .....(2)
.....(2)