Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
if 600.0 grams HF reacts with excess SiO2, how many grams of H2O are produced? Use the following mol...
Questions

Mathematics, 25.03.2021 18:50

Mathematics, 25.03.2021 18:50

Biology, 25.03.2021 18:50

Biology, 25.03.2021 18:50




Mathematics, 25.03.2021 18:50

Mathematics, 25.03.2021 18:50


Chemistry, 25.03.2021 18:50

Mathematics, 25.03.2021 18:50

Mathematics, 25.03.2021 18:50




English, 25.03.2021 18:50

Mathematics, 25.03.2021 18:50

Mathematics, 25.03.2021 18:50

Social Studies, 25.03.2021 18:50


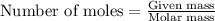 ......(1)
......(1)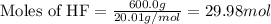
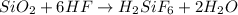
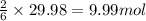 of water
of water