
Chemistry, 17.06.2021 05:00 shashi2728
The reaction (image below) can be abbreviated as HCl → H+ + Cl-. How is this apparently important omission justified?
A)
It isn't justified, and the abbreviation is an error.
B)
It's understood that H+ doesn't exist on its own in water, and image are both aqueous.
C)
It's generally understood that H3O+ is a hypothetical intermediary.
D)
Because water isn't important to the reaction
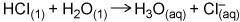

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
The reaction (image below) can be abbreviated as HCl → H+ + Cl-. How is this apparently important om...
Questions

Chemistry, 18.10.2019 08:00


History, 18.10.2019 08:00


Mathematics, 18.10.2019 08:00

Mathematics, 18.10.2019 08:00





Mathematics, 18.10.2019 08:00

Mathematics, 18.10.2019 08:00


Mathematics, 18.10.2019 08:00

Mathematics, 18.10.2019 08:00


History, 18.10.2019 08:00

Physics, 18.10.2019 08:00

History, 18.10.2019 08:00

Biology, 18.10.2019 08:00



