
Chemistry, 17.06.2021 05:00 tayler6289
Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell potential for a voltaic cell made from these two systems. (HELP ASAAAP)
A)
1.68 V
B)
0.78 V
C)
–1.68 V
D)
–0.78 V
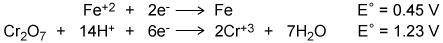

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell p...
Questions


Mathematics, 19.07.2019 16:00

Mathematics, 19.07.2019 16:00

World Languages, 19.07.2019 16:00

Mathematics, 19.07.2019 16:00

World Languages, 19.07.2019 16:00

Computers and Technology, 19.07.2019 16:00


History, 19.07.2019 16:00


Business, 19.07.2019 16:00


Biology, 19.07.2019 16:00

Computers and Technology, 19.07.2019 16:00


History, 19.07.2019 16:00


Biology, 19.07.2019 16:00




