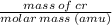Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
One atom of chromium (Cr) has a mass of 52.00 amu. How many chromium atoms does it take to equal a m...
Questions


Arts, 03.02.2021 17:30


English, 03.02.2021 17:30

Mathematics, 03.02.2021 17:30






Mathematics, 03.02.2021 17:30

World Languages, 03.02.2021 17:30



History, 03.02.2021 17:30

Mathematics, 03.02.2021 17:30



History, 03.02.2021 17:30