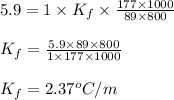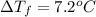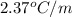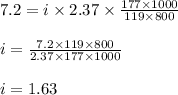Chemistry, 16.06.2021 15:40 PersonPerson13260
When of alanine are dissolved in of a certain mystery liquid , the freezing point of the solution is less than the freezing point of pure . Calculate the mass of potassium bromide that must be dissolved in the same mass of to produce the same depression in freezing point. The van't Hoff factor for potassium bromide in .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Write skeleton equations for the following reactions c. aluminum(s)+copper(i) chloride(aq) > aluminum chloride(aq)+copper(s)
Answers: 1

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

You know the right answer?
When of alanine are dissolved in of a certain mystery liquid , the freezing point of the solution is...
Questions

Mathematics, 08.10.2019 23:30



Mathematics, 08.10.2019 23:30

English, 08.10.2019 23:30








Computers and Technology, 08.10.2019 23:30

Computers and Technology, 08.10.2019 23:30






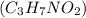 are dissolved in 800.0 g of a certain mystery liquid X, the freezing point of the solution is
are dissolved in 800.0 g of a certain mystery liquid X, the freezing point of the solution is 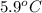 lower than the freezing point of pure X. On the other hand, when 177.0 g of potassium bromide are dissolved in the same mass of X, the freezing point of the solution is
lower than the freezing point of pure X. On the other hand, when 177.0 g of potassium bromide are dissolved in the same mass of X, the freezing point of the solution is 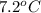 lower than the freezing point of pure X. Calculate the van't Hoff factor for potassium bromide in X.
lower than the freezing point of pure X. Calculate the van't Hoff factor for potassium bromide in X.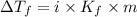
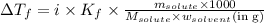 ......(1)
......(1)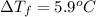
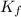 = freezing point depression constant
= freezing point depression constant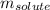 = Given mass of solute (alanine) = 177. g
= Given mass of solute (alanine) = 177. g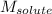 = Molar mass of solute (alanine) = 89 g/mol
= Molar mass of solute (alanine) = 89 g/mol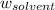 = Mass of solvent = 800.0 g
= Mass of solvent = 800.0 g