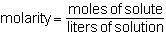Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
In a group assignment, students are required to fill 10 beakers with 0.720 M CaCl2. If the molar mas...
Questions

Biology, 17.06.2021 19:20







Spanish, 17.06.2021 19:20

Mathematics, 17.06.2021 19:20

Chemistry, 17.06.2021 19:20

Mathematics, 17.06.2021 19:20

Mathematics, 17.06.2021 19:20




English, 17.06.2021 19:20

Physics, 17.06.2021 19:20