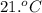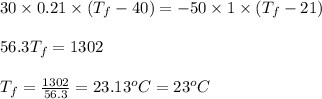Chemistry, 16.06.2021 08:00 Shadow0202
A 30. g sample of Aluminum was heated to 40. 0C and placed in a calorimeter containing 50. g of water at 21 0C. What is the final temperature of the aluminum-water system if the cAl = 0.21 cal/g0C and cwater = 1.0 cal/ g 0C.
Write the complete equation you will use. 1 point
Substitute the values in the equation in step 1 . 1 point
Report the math answer with 2 sig figs and the correct unit. 1 point

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
A 30. g sample of Aluminum was heated to 40. 0C and placed in a calorimeter containing 50. g of wate...
Questions

Mathematics, 29.11.2020 14:00


English, 29.11.2020 14:00


Mathematics, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00

English, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00


History, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00


Physics, 29.11.2020 14:00


Geography, 29.11.2020 14:00

Social Studies, 29.11.2020 14:00

Physics, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00

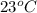

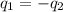
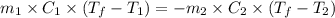 ......(1)
......(1)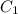 = specific heat of aluminium =
= specific heat of aluminium = 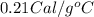
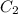 = heat capacity of water =
= heat capacity of water = 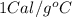
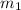 = mass of aluminium = 30. g
= mass of aluminium = 30. g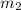 = mass of water = 50. g
= mass of water = 50. g = final temperature of the system = ?
= final temperature of the system = ?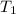 = initial temperature of aluminium =
= initial temperature of aluminium = 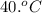
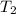 = initial temperature of the water =
= initial temperature of the water =