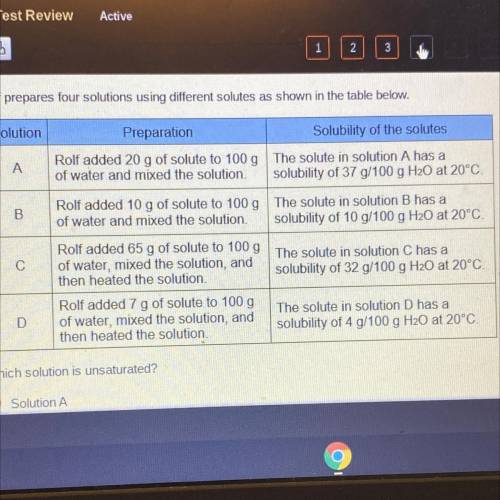
Rolf prepares four solutions using different solutes as shown in the table below.
Solution
Pr...

Rolf prepares four solutions using different solutes as shown in the table below.
Solution
Preparation
Solubility of the solutes
A
Rolf added 20 g of solute to 100 g The solute in solution A has a
of water and mixed the solution solubility of 37 g/100 g H20 at 20°C!
B
Rolf added 10 g of solute to 100 g The solute in solution B has a
of water and mixed the solution. solubility of 10 g/100 g H20 at 20°C.
С
Rolf added 65 g of solute to 100g The solute in solution C has a
of water, mixed the solution, and
then heated the solution,
solubility of 32 g/100 g H20 at 20°C
D
Rolf added 7 g of solute to 100 g
of water, mixed the solution, and
then heated the solution
The solute in solution D has a
solubility of 4 g/100 g H20 at 20°C.
Which solution is unsaturated?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
Questions


Mathematics, 13.02.2021 21:30




Mathematics, 13.02.2021 21:30

French, 13.02.2021 21:30

Social Studies, 13.02.2021 21:30



Advanced Placement (AP), 13.02.2021 21:30

History, 13.02.2021 21:30

History, 13.02.2021 21:30

Biology, 13.02.2021 21:30

SAT, 13.02.2021 21:30


Mathematics, 13.02.2021 21:30

Mathematics, 13.02.2021 21:30




