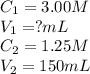Chemistry, 15.06.2021 20:30 lanettejohnson355
A stock solution of calcium sulfate, CaSO4 has a concentration of 3.00 M. The volume of this solution is 150 mL. What volume of a 1.25 M solution could be made from the stock solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
A stock solution of calcium sulfate, CaSO4 has a concentration of 3.00 M. The volume of this solutio...
Questions


English, 20.07.2019 08:10





Mathematics, 20.07.2019 08:10

Mathematics, 20.07.2019 08:10



Mathematics, 20.07.2019 08:10





Computers and Technology, 20.07.2019 08:10

Geography, 20.07.2019 08:10



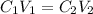 ....(1)
....(1)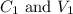 are the concentration and volume of stock solution.
are the concentration and volume of stock solution.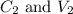 are the concentration and volume of diluted solution.
are the concentration and volume of diluted solution.