
Chemistry, 15.06.2021 19:10 nuggetslices
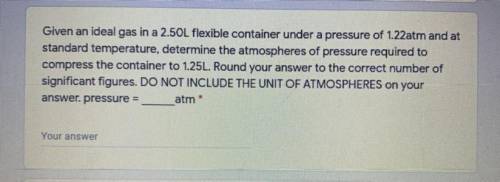
Given an ideal gas in a 2.50L flexible container under a pressure of 1.22atm and at
standard temperature, determine the atmospheres of pressure required to compress the container to 1.25L. Round your answer to the correct number of
significant figures

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1


Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
Given an ideal gas in a 2.50L flexible container under a pressure of 1.22atm and at
standard temper...
Questions

Mathematics, 07.07.2019 17:30

Mathematics, 07.07.2019 17:30

Mathematics, 07.07.2019 17:30


Mathematics, 07.07.2019 17:30


Social Studies, 07.07.2019 17:30


History, 07.07.2019 17:30

Mathematics, 07.07.2019 17:30

Mathematics, 07.07.2019 17:30

History, 07.07.2019 17:30






History, 07.07.2019 17:30





